A Beginners Guide To Hydrogen Fuel-Cell Electric Vehicles

Here’s all YOU need to know about hydrogen fuel cell electric vehicles, explained in (hopefully) the simplest way possible.
While electric vehicle are certainly all the rage these days, the overwhelming majority of them are to be of the battery-powered variety. But did you know that there is actually another way out there to power an EV, with the use of a hydrogen fuel cell?
In fact, production versions of hydrogen fuel cell electric vehicles (henceforth known as HFCVs to keep things concise) have actually been on the market for well over a decade now, with it offering some distinct benefits over the conventional battery-powered electric vehicles (BEV) as well.

Unfortunately, due to a confluence of factors (which will be discussed further below), market penetration remains elusive for HFCVs, and this has thus lead to many still being in the dark regarding this rather interesting zero-tailpipe emissions motoring alternative. So hence is born this piece here aiming to provide an answer for just a few of the more pressing questions regarding this particular alternative fuel vehicle type, in hopefully the simplest way possible!
1. What Is A Hydrogen Fuel Cell EV?
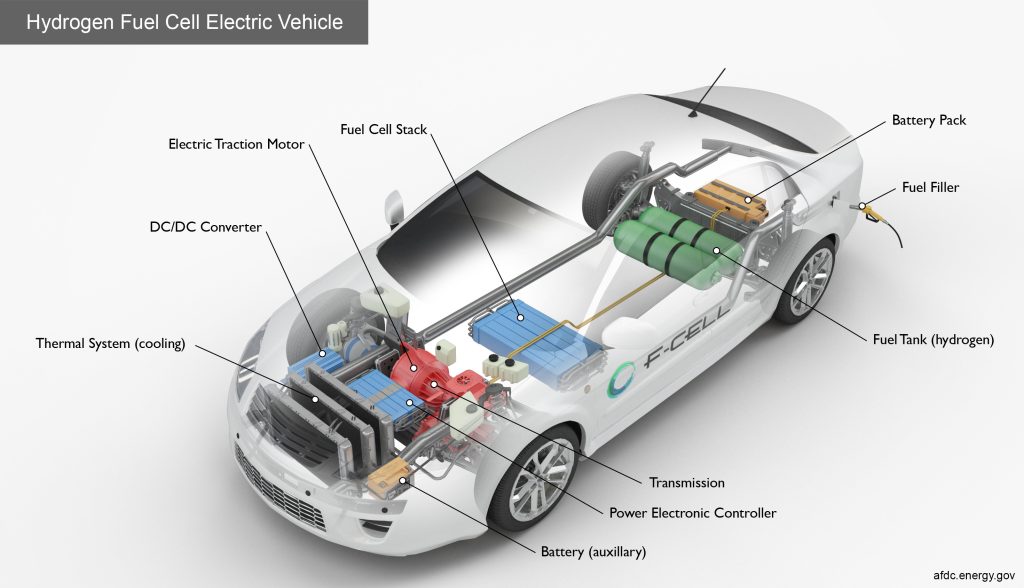
Very simply here, the propulsion part of a HFCV is actually much the same as in a regular battery-powered EV, as driving the wheels of a HFCV is still to be an electric motor. The difference between a BEV and a HFCV only starts from how the motors are powered, with electricity to be generated on-board a HFCV from a hydrogen fuel cell stack instead of coming from stored electricity in a battery with a BEV.
It is nevertheless worth noting though that there is still usually to be a high-voltage drive battery involved in most production HFCVs, albeit in a smaller size, to store energy generated from regenerative braking and provide supplemental power to the motor when required.
2. How Does A Hydrogen Fuel Cell Work?
Without going too technical into the chemistry of it, a hydrogen fuel cell essentially initiates a redox chemical reaction between hydrogen and oxygen to generate energy. The full chemical equation of this reaction for the chemistry nerds is as follows: 2H₂ + O₂ ➔ 2H₂O + energy. Hydrogen in a HFCV is obtained from the on-board high pressure storage tank, while oxygen comes from the ambient air.

3. Who Currently Produces Hydrogen Fuel Cell EVs?
Current makers of HFCVs are mainly of the Asian persuasion, with Toyota all but deemed the standard bearer of this alternative fuel option, and its Mirai sedan (incidentally the world’s first mass-produced HFCV back in 2014) likely being the most well-known example of production HFCV around.

The other notable players presently in this field meanwhile include Hyundai and Honda. Hyundai currently produces the Nexo HFCV e-SUV and has dabbled in hydrogen fuel cells for heavy haulage with its XCIENT line of lorries (in addition to continually teasing the imminent arrival of its cyberpunk hydrogen-powered N Vision 74 supercar), while Honda has just recently launched its CR-V e:FCEV hydrogen fuel cell PHEV in North America.

Many automakers have in any case dabbled with HFCV development in the recent past, with Mercedes-Benz, BMW and General Motors among the more notable to previously reveal working prototypes. Fun fact here too upon mention of GM is that the first-ever HFCV passenger vehicle was actually Chevrolet Electrovan concept, that came out all the way back in 1966.

4. Why Are The Benefits Of Hydrogen Fuel Cell EVs?
As per the chemical equation for electricity generation using a hydrogen fuel cell shows, a HFCV emits zero tailpipe emissions. Or at least no toxic emissions anyway, because the only byproduct of the electricity generation process from a hydrogen fuel cell is to be H₂O, aka water! This therefore makes HFCVs a greener alternative to the current carbon (and NOx etc.) emitting ICE cars around.

In terms of its benefits against BEVs on the other hand, the main advantage HFCVs hold is that refilling its high-pressure tanks tends to only take about the same as refuelling a petrol or diesel-powered car, instead of the at least half hour wait needed to top up the batteries on a BEV when connected to the highest output of (commercially-available) DC chargers.

HFCVs have also been shown to operate more effectively at greater temperature extremes relative to BEVs, with batteries not operating most efficiently when too hot (high internal resistance) or too cold (slow chemical reaction). The biggest (yet least talked about) technical advantage HFCVs hold over BEVs however is for the 5 times greater relative energy density of compressed hydrogen to negate the effects of mass compounding from a lithium-ion battery pack, which in very simple terms means that HFCVs do not add as much weight as BEVs would (in the form of additional heavy batteries) to travel increasingly further distances or haul heavier loads.

5. What Are The Pitfalls Of Hydrogen Fuel Cell EVs?
One of the biggest current societal barriers to wider HFCV adoption is to be the lack of infrastructural support for hydrogen refuelling, with this technically being a Catch-22 scenario from the lack of HFCVs available. It should go without saying also that (most non-PHEV) HFCVs also do not have the possibility of charging at home, with the current rising fuel cost for hydrogen (due to rising operation costs and inflation) further hampering the attractiveness of HFCVs as a commercially-viable product.

From a technical perspective too, HFCVs have a couple of significant drawbacks relative to BEVs. The first of these is for HFCVs have a very low well-to-wheel efficiency, which sees it to consume about two to three times more electricity for the same distance as a BEV. This inefficiency primarily comes from the many energy-intensive processes required to produce pure hydrogen (usually from chemically cracking it from a compound like methane, CH₄) and then converting it back to electricity in the fuel cell.
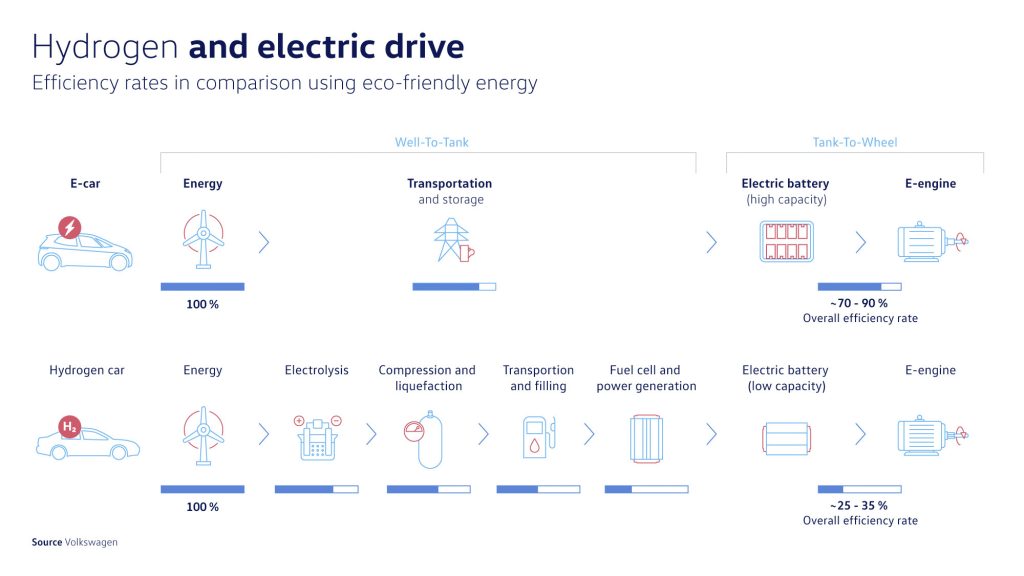
There is further the more practical point of HFCVs needing volumetrically-bulky high-pressure tanks to store said hydrogen, which in some cases may take up more space within a vehicle than what current batteries occupy under the floors of most BEVs.

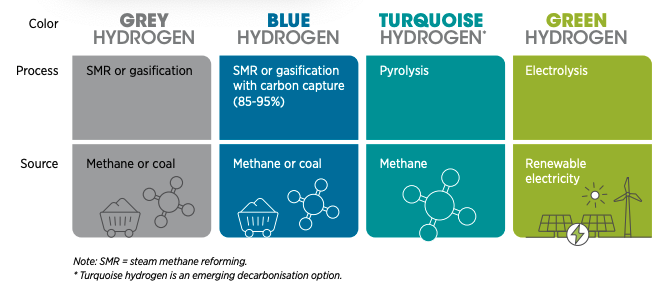
Environmentalists would tend to point out as well that hydrogen production (at present at least) is not exactly all that green, as the most common method currently used today, steam methane reforming, uses fossil fuels in the form of natural gas and releases CO₂ as a byproduct. Ecologically-sound production methods for H2 by using renewable energy to split water into hydrogen and oxygen are nevertheless available, but the issue right now is for these methods to not be economically feasible.
6. Alternative Applications Of Hydrogen In Cars
In ending this brief explainer on HFCVs here on a slight tangent, the use of hydrogen in cars is not actually limited to feeding a fuel cell that powers an EV. Such is as Toyota have long since been demonstrating with its experimental Corolla race car, hydrogen can also be burnt in an internal combustion engine as a form of fuel with zero carbon emissions.

Hydrogen combustion is currently seen as a way for the internal combustion engine to remain relevant in the eco-conscious future, with Toyota once again spearheading this movement by hinting that its next-generation of ICE will be made compatible with a host of carbon-neutral fuels, which includes liquid hydrogen.





