Solid-state batteries might make a return in electric cars

Chinese manufacturer, Dongfeng has delivered a new batch of test vehicles that use solid-state batteries (SSB). This news from carnewschina.com, states that the Chinese company has decided to go back to using solid-state batteries. Back in the day, electric cars used to mainly use solid-state lithium-ion batteries. However, it was later discovered that liquid electrolyte was essential in lithium-ion batteries as they were safer and more durable for use in electronics and vehicles.
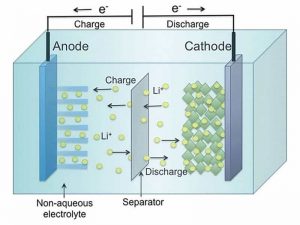
In case you didn’t know, a lithium battery cell consists of several parts. There’s a positive terminal (cathode), a negative terminal (anode), a separator between the two, and a medium through which lithium ions can move from one terminal to the other (electrolyte). When charging the battery, lithium ions move from the cathode to the anode while their free electron (the ‘electricity’) moves in the opposite direction. During discharge, the process is reversed.
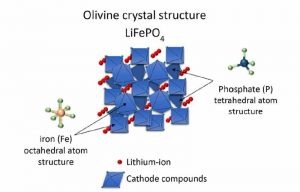
A batteries capacity is determined by the number of lithium-ions that can be stored. For example, on the cathode side, Lithium-Iron-Phosphate (LFP) batteries have a dense and stable crystalline structure that stores lithium-ions in tubes, meaning that the ions can move in only one direction. So, moving to the other terminal goes relatively slowly.
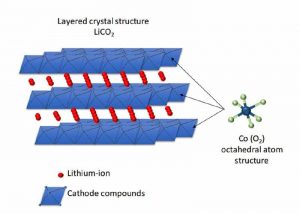
Meanwhile, Nickel-Cobalt-Manganese (NCM) batteries store lithium in planes, meaning two dimensions for moving around. This chemistry stores more lithium in the same volume, moving around faster. On the downside, when lithium moves to the anode, the NCM-structure becomes unstable and would collapse if all lithium moved away. Some lithium has to stay behind, reducing the capacity advantage by a bit.
The reason why solid-state batteries are coming back has everything to do with the anode side. LFP and NCM batteries both use carbon as an anode. Although, carbon stores lithium well, other materials such as silicon have a larger storage capacity. This is why many batteries used to use lithium-metal compounds on the anode side to store more lithium.
Early states of the rechargeable battery involved the lithium-metal anode being replaced with carbon. The only problem was carbon stored fewer lithium-ions than lithium-metal which meant reduced capacity and the batteries were bigger and heavier.
The first solid-state battery appeared on the market over ten years ago and consisted of solid polymer electrolyte. Combined with an LFP cathode and Li-metal anode, this battery was commercialized by BlueSolutions and has been on the market since 2011. It was used in the Bollore Bluecar car-sharing program in Paris and is currently available on some Mercedes-Benz buses. This battery also had its fair share of problems, like the fact that it had an operating temperature of 80 degrees Celsius and it had a low capacity.
Battery manufacturers have basically settled with only two options. Either a variation of the old solution, lithium-metal anode, and lithium oxide (Li-OH) solid electrolyte, or the lithium-sulphur (Li-S) battery.
For the Li-OH option, this new innovation involves the use of lithium oxide as a solid electrolyte. It uses the same chemistries like the LFP and NCM on the cathode side, while the anode is lithium-metal. The advantage of this option is the fact that it doesn’t have to be fully solid. Although it has a solid electrolyte, it still contains a small amount of liquid electrolyte as well. This semi-SSB batteries will be route of choice for many Chinese manufacturers. They can introduce semi-SSB’s and slowly reduce the amount of liquid electrolyte present. It is estimated that SSB’s using Li-OH will likely be more than at least 5 years away.
Next is the lithium-sulphur battery. Toyota is already developing a lithium-sulphur SSB with a product reveal scheduled at the Tokyo Olympics. However, because of how difficult a Li-S battery is to manufacture, they have failed to deliver. Despite how difficult it Is, lithium-sulphur has the highest storage capacity of known materials. If successfully done, the chemistry involved could bring a battery with up to 500% more capacity.
The biggest issues about its development are related to the controlled environment required. If Li-S comes into contact with water, it will produce hydrogen sulfide (H2S) which is harmful to humans. This also means that, for the battery to be used safety, severe waterproofing must be done. The material is highly sensitive and particles in our matter can also cause issues within the battery. This has led to the battery being produced in a dry and clean room, which is strictly controlled. Information coming from Toyota is that they assemble Li-S cells by hand inside a glove box (small “aquarium” with sealed gloves attached, in which the operator can put his hands) with a 100% Argon atmosphere. Argon is a mostly non-reactive element. This production method is not scalable, and it would be very costly to make an EV battery this way.

In conclusion, solid state batteries might be the next step in EV technology. Several companies have already started to market their first SSB’s, but we still don’t know if it will live up to our expectations. Not to mention, these batteries aren’t going to be cheap until scientists find a way to make it affordable. All we can do now is wait and see.




